Making of fused quartz and fused silica
As the title implies, there are two distinctive ways to produce fused silica. One starts with high purity quartz, or other silicon dioxide containing minerals that are fused using various heat sources. This material class is termed fused quartz. The other is called fused silica and starts with gaseous silicon containing chemicals (e.g. SiCl4) that are burned in the presence of oxygen to form silicon dioxide.
Fused quartz
Electric fusion
Electric fusion is the most commonly used melting process for manufacturing quartz glass. Two methods of electric fusion can be used:
- Continuous fusion:
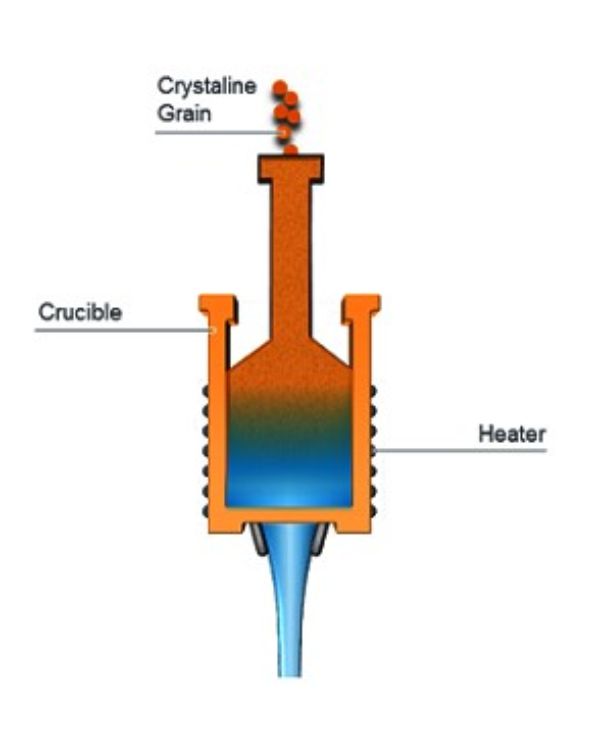
In the continuous method, quartz sand is poured into the top of a vertical melter that consists of a refractory metal crucible surrounded by electric heating elements. The interior is maintained in a neutral or slightly reducing atmosphere that keeps the silica from reacting with the refractory metal. The melted material exits the bottom orifice of the crucible, which is shaped to produce rods, tubes, plates or other products of various dimensions. - Batch or boule fusion:
In the batch fusion method, a large quantity of raw material is placed inside a refractory lined vacuum chamber which also contains heating elements. Although this method has historically been used to produce large single boules of material, it can also be adapted to produce much smaller, near-net shapes.
Purity is mostly determined by the extent of refinement of the raw material and the process used. Heraeus uses only very highly refined quartz sand coupled with rigorous quality control to make its products.
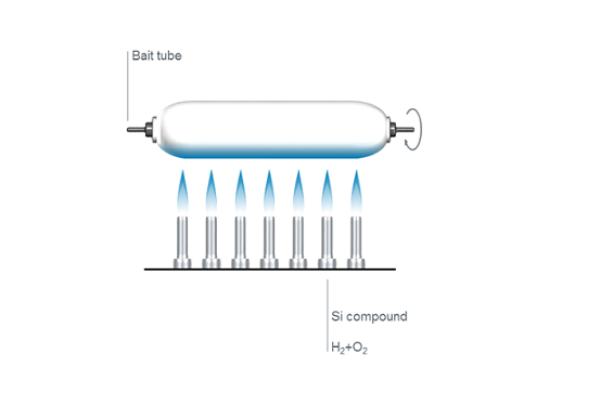
Flame fusion
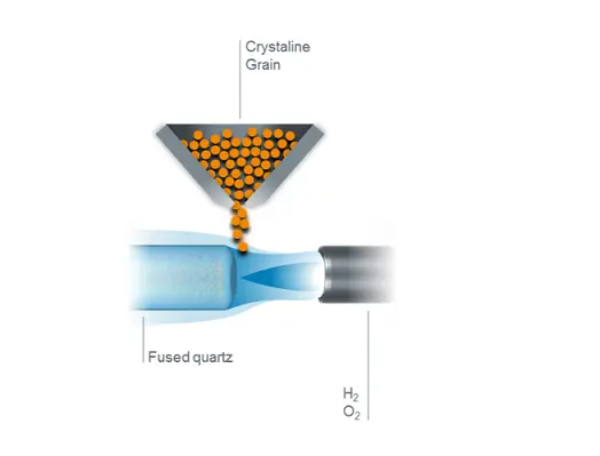
Historically, the first method of producing fused quartz was by small-scale fusion of quartz crystals in a flame. Heraeus chemist Richard Küch first began fusing quartz rock crystal in a hydrogen/oxygen (H2/O2) flame more than 100 years ago. Heraeus has been producing quartz glass on an industrial scale with this process ever since.
Today, flame fused quartz is manufactured on a large scale by a continuous process in which highly refined quartz sand is fed through a high temperature flame and deposited on the surface of a melt contained in a tank lined with refractory material. The viscous melt is withdrawn slowly through a die in the bottom of this tank, and it solidifies in a shape determined by the die. In this way it is possible to produce an ingot of transparent fused quartz of the required cross-section (round, rectangular or hollow), which is cut off at intervals, and removed for further processing.
Fused silica
In this process, the silicon containing precursors (e.g. Silicon Tetra Chloride; STC) burn in the presence of oxygen to form nano-particles of silicon dioxide, also called soot. Precise production and refinement of precursors leads to exceptionally high purity and the resulting fused silica has a very low metallic impurity content.
As the production process involves chemical vapors (silicon containing precursors), it is called chemical vapor deposition (CVD). There are two types of CVD processes, one where the deposited nano-particles directly melt to a condensed fused silica layer, and another, called vitrification, where the soot accumulates and condenses to transparent fused silica.
One-step fused silica production
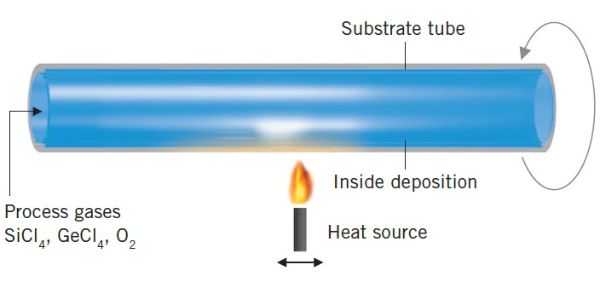
Chemical vapor deposition (CVD)
For the production of optical fiber core rods, deposition of fused silica with a defined refractive index is done inside of fused silica tubes. A carrier gas brings the chemicals. CVD processes are differentiated according to the type of heat source used, that triggers the reaction to form soot. The heat source is either a flame (MCVD), a furnace (FCVD) or a plasma (PCVD). All gases that have not reacted are treated in a scrubber.
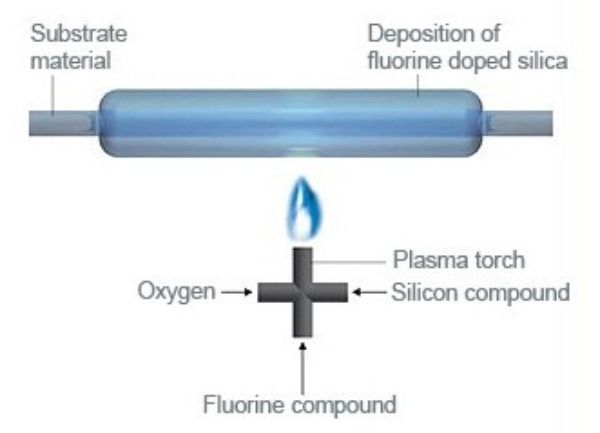
Plasma outside deposition (POD)
In this process a plasma heat source is used to “burn” chemical precursors and deposit a thin glass layer on a rotating target. The target can be a tube or a solid rod, it is not necessarily round. Because of the high temperature of the plasma, this process is best suited to produce fluorine doped fused silica. The achievable maximum content of fluorine in silica is a function of deposition temperature. There is a limit to the maximum fluorine content, because fluorine also etches away fused silica. The higher the fluorine content in the gases, the slower the deposition is.
POD is typically used to produce highly fluorine doped silica which has a lower refractive index than undoped fused silica. This difference in refractive index is needed for optical fibers. The available products are highly fluorine doped tubes and rods, but Heraeus offers it as a service as well.
Two-step fused silica production
In this process, soot is deposited on a rotating bait rod (outside vapor deposition; OVD) or on the end of a rod that is pulled upwards (vapor axial deposition; VAD). The soot accumulates and forms a porous body with a density that is less than 25 % of fused silica. This porous body is then vitrified to transparent fused silica in a consecutive step.
Because of its high surface, it is easy to dope the porous soot body. In the fiber optic industry hydrogen is substituted with chlorine in a dehydration step before vitrifying the soot body.